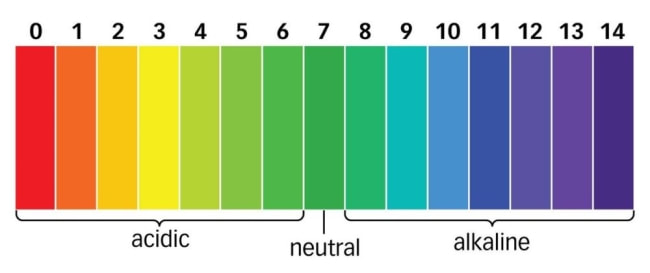
In chemistry, the pH (potential hydrogen) scale is used to measure the concentration of hydrogen ions. The pH scale is used in horticulture to determine if a soil, water source, or nutrient solution is neutral, acidic, or alkaline (basic). On the pH scale, a reading of 7 is considered neutral, all readings above 7 are considered alkaline and all readings below 7 are considered acidic. The pH scale is an exponential logarithmic scale and every number on the pH scale represents an increase or decrease of ten fold. For example, a pH value of 4 is ten times more acidic than a pH value of 5 and 100 times more acidic than a pH value of 6. The same holds true for readings above 7. For example, a pH value of 10 is ten times more alkaline than a pH value of 9 and 100 times more alkaline than a pH value of 8. An understanding of the pH scale is important for horticulturalists because every essential element used by plants has an ideal pH range in which it can be absorbed. If the pH fluctuates too far from the ideal range a nutrient “lockout” may occur and thereby cause nutrient deficiencies and hinder the vibrance within a greenhouse.
The Ideal pH Range
For soil gardening, the ideal pH range falls just below neutral, in the 6-7 pH range, with the maximum absorption around 6.3-6.8. Why and how did this become the ideal pH range for nutrient uptake? The answer lies in the complex microscopic world of microorganisms living in and around the rhizosphere (the region of soil affected by a plant’s root secretions). A slightly acidic pH is ideal for the reproduction and functioning of the beneficial microorganisms that help break down organic matter into soluble nutrients. When pH values drop below 6 there is a significant decrease in the availability of nitrogen, potassium, sulfur, calcium, and magnesium but the largest decrease is in the availability of phosphorus. Low pH values are the main cause of a phosphorus deficiency. Many horticulturalists experience phosphorus deficiencies not because there is an inadequate amount of phosphorus in the soil but instead because the pH of the soil has become too acidic.
Hydroponic gardeners will find the ideal pH for nutrient absorption is slightly more acidic than for soil gardening. In hydroponics there is less of a dependency on the microbial life which is normally harbored in the soil. Hydroponic nutrients are already broken down into a soluble form. This bypasses many of the functions performed by the beneficial microbes thus changing the optimal pH range for nutrient absorption. The ideal pH range for hydroponic gardening falls in the 5-6 range, with maximum absorption around 5.5-5.8. Unlike soil, the pH value of a hydroponic solution can fluctuate rapidly. This is why it is so important to monitor the pH of the solution daily, if not more often.
Testing the pH
There are two common methods to test the pH value of a soil: using a pH meter with a soil specific probe or creating a soil solution for testing. Soil specific pH meters have a probe that is inserted directly into the soil to measure the pH value. These meters can be either digital or analog and may require calibration depending on the particular meter. Since it is the most sensitive part of the equipment, more expensive meters will have a removable probe that can be replaced.
Another method is to create a liquid solution out of a soil sample for pH testing. To create a soil solution, a horticulturalist should take a sample of soil (a couple of handfuls) and allow it to dry completely. Weigh out 20g of the dried, collected soil and place it in a small jar. Add 100ml of distilled water to the small jar then shake it for at least five minutes. Allow the concoction to settle overnight and then shake it up again the following morning. Allow the solution to settle for fifteen minutes and then strain the sample into a clean container. To test the pH value of this liquid soil solution, or any other liquid sample (water, hydroponic nutrient solution, insecticide or pesticide solution, etc.), a horticulturalist may choose from the following options:
Litmus Paper
Litmus paper can be purchased at most pool supply, aquarium, or hydroponic stores and is one of the oldest and most common methods for testing the pH of an aqueous solution. Litmus paper is paper that has been treated with a specific indicator; a mixture of multiple dyes obtained from lichens that turn red in response to acidic conditions and blue under alkaline conditions. The advantages of Litmus paper are cost (very inexpensive in relation to other pH testing methods) and no calibration is required. A disadvantage of Litmus paper is obtaining accurate readings may be difficult. Some people find it tough to match a specific color to the corresponding pH value. Litmus paper is a great choice for finding the general pH range of a solution but should be avoided when trying to find a precise pH value down to a tenth of a point.
Liquid pH Test Drops
Liquid pH test drops usually come in a small dropper bottle and work similarly to litmus paper. A sample of the solution is placed in a small test tube. A certain number of drops (usually 1-3 depending on the manufacturer’s suggestion) are then added to the test solution and shaken vigorously. The pH indicators in the test drops cause the solution to change color. The color of the solution is matched to the pH scale and value provided on the packaging of the test drops. Advantages of liquid test drops are the same as litmus paper: low cost and no need to calibrate. Many users find the liquid pH test drops’ corresponding colors easier to match than the litmus paper test. Again, if accurate measurements down to a tenth of a point are needed liquid pH test drops would not be the preferred method.
Analog pH Meters
These beauties are being used less frequently by greenhouse horticulturalists. Once thought to be the best thing since sliced bread, analog pH meters, although extremely reliable and accurate, have been replaced by less expensive (and almost just as accurate) digital meters. Analog pH meters generally have a small sampling cup to place the solution in and a small window with a needle indicator which displays the pH value. Most quality analog pH meters give accurate readings to a tenth of a point and display accurate readings immediately.
Digital pH Meters
Digital pH meters are probably the most common method used by hobbyist hydroponic growers. Digital pH meters come in many different sizes, shapes, and functionalities. As technologies advance, digital pH meters are becoming less expensive and more accurate. With quality starting models priced around $40 there is really no excuse for a hydroponic gardener to be without a digital pH meter. Most digital pH meters are accurate to a tenth of a point and are consistently reliable (assuming they are maintained properly). Disadvantages of digital meters are that they require routine cleaning, batteries, and calibration. Digital meters may also require some patience as it may take a couple of minutes before they will display an accurate reading.
Digital pH Meters with Temperature Compensation
Horticulturalists who want accurate pH readings immediately should choose a digital pH meter with built-in temperature compensation. Unless the meter is equipped with temperature compensation the temperature difference between the nutrient solution and the glass probe itself will create a delay in producing accurate readings until the probe and solution reach the same temperature. Meters with temperature compensation automatically take into account the variance in temperature between the probe and the solution and give instantaneous, accurate readings regardless of temperature. These high tech meters are more expensive than standard digital meters and also require cleaning, batteries, and calibration.
Making pH Adjustments
After the pH of the soil or solution has been tested you may find that the pH value is off and needs some adjustment. For soil that is too acidic dolomite lime is a great, fast working additive. Oyster shell can also be added to raise the pH of an acidic soil, although it usually takes a bit longer to affect the pH. A less common problem is soil that is too alkaline. The best way to combat this issue over the long term is to add organic matter to your soil. Mulch and compost will help but the best additives are organic materials that are already naturally acidic like sphagnum peat moss. If you have alkaline soil that needs rectifying immediately, the best option is sulfur in either its elemental form or in an aluminum sulfate form. The aluminum sulfate form will work the fastest, creating acidity as soon as it dissolves into the soil.
To make adjustments to a hydroponic nutrient solution or your water source there are liquid pH buffers available. These buffers are usually sold under the generic name of pH Up and pH Down. Although a frugal horticulturalist could substitute vinegar or lemon juice for pH Down, baking soda or similar household items should never be substituted for pH Up. This is because of the excess of calcium that could be added to the soil or solution which would bond to magnesium and almost inevitably cause a magnesium deficiency. Buffers (pH Up and pH Down) designed for horticultural usage are void of calcium or other bonding elements and are usually composed of potassium molecules.
When making pH adjustments to your solution or water source it is extremely important to remember that pH is an exponential scale. In other words, it will take much more pH Up buffer to raise a pH value from 4 to 5 than it would to raise it from 5 to 6. This means as you are adjusting a solution you will have to incrementally adjust the amount of buffer you are adding to compensate properly. The best method I have found is to add very small amounts of the buffer and retest often, being careful not to overshoot your target.
The pH value of a soil, water source, or hydroponic solution is a determining factor in the population of microbial life within the medium and is directly linked to nutrient uptake. Testing, adjusting, and maintaining the pH value is imperative to maximizing virtually all plant functions and can be a fun way for the hobbyist greenhouse horticulturalist to become more technologically advanced. Not all gardeners need a digital pH meter with temperature compensation to test the pH range in their garden. Those devices are probably best suited for the serious hydroponic gardener. However, a dollar or two for some litmus paper could allow hobbyist horticulturists to increase their knowledge of the pH scale, how pH affects their garden, and possibly help to prevent problems caused by a pH imbalance.
Eric Hopper is the MyGardenAndGreenhouse.com editor. He resides in Michigan’s beautiful Upper Peninsula where he enjoys gardening and pursuing sustainability.
Related Articles & Free Email Newsletter
Early Growth Fertilizer Regiments for Propagation & Vegetative Stages
The Essential Elements of Hydroponic Nutrients




Comment here